Thermal analyses of some fluoroquinolone pharmaceutical compounds in comparison with molecular orbital calculations
Abstract
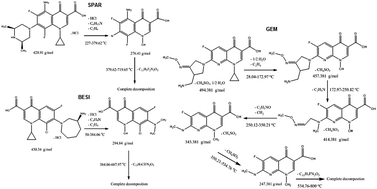
The thermal stability, purity and compatibility of some fluoroquinolones, including sparfloxacin HCl (SPAR), besifloxacin HCl (BESI), gemifloxacin mesylate (GEM), and their excipients have been successfully studied using thermal analysis techniques. Thermogravimetric analysis (TG), derivative thermogravimetry (DTG), differential thermal analysis (DTA) and differential scanning calorimetry (DSC) were used to study the thermal behavior of these drugs. The melting points of SPAR, BESI and GEM were 261.33, 270.04 and 287.13 °C, respectively. The melting point values show satisfactory results in comparison with those obtained by using the official method. The Ozawa method was employed to determine the activation energy values of the thermal decompositions of these drugs. Comparison of the activation energy values suggests the following sequence of thermal stability: SPAR > BESI > GEM. Semi-empirical molecular orbital calculations (PM3 method) were used to confirm the thermal decomposition steps and the order of stability of these drugs. The purity value of SPAR (99.98%) was determined by DSC indicating similarity to that found by the reported method (99.95%). The results obtained are useful for these drugs, since they show good interpretations for their thermal decomposition and stability.


 Please wait while we load your content...
Please wait while we load your content...