Evaluation and comparison of N-cycloalkylformylated chitosan bis(arylcarbamate)s as chiral selectors for enantioseparation†
Abstract
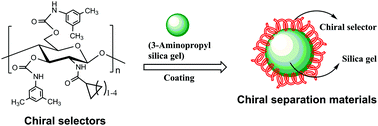
In order to systematically investigate the structural dependence on the properties of N-cycloalkylformylated chitosan bis(arylcarbamate)s, 3,5-dimethylphenylcarbamates of N-cyclopropylformylated, N-cyclobutylformylated, N-cyclopentylformylated and N-cyclohexylformylated chitosans were prepared as chiral selectors in the present study. Since both the molecular weights of chitosan and the substituents at the 3- and 6-positions of the phenylcarbamates are identical, the influences of the substituent at the 2-position on the solvent tolerability and enantioseparation performance of the chiral selectors were specifically compared and discussed. It was found that the solvent tolerability and enantioseparation performances of the chiral selectors obviously differed with the variation of the substituent at the 2-position of the glucosamine skeleton. Although all the chiral selectors generally showed satisfactory tolerability in ethyl acetate and acetone, the chiral selector with a three-membered ring exhibited much more preferable tolerability against tetrahydrofuran than the others with a four-, five- or six-membered ring. Enantioseparation results revealed that most of the chiral selectors exhibited powerful chiral recognition and enantioseparation abilities, and the chiral selector with a five-membered ring exhibited the best enantioseparation performance. The corresponding coated-type chiral stationary phases prepared from these chitosan-based chiral selectors were able to be complementary with each other and applied as promising chiral separation materials for enantiomeric separations.


 Please wait while we load your content...
Please wait while we load your content...