Palladium(ii)–palladium(ii) bonding in two open clamshell dinuclear complexes†
Abstract
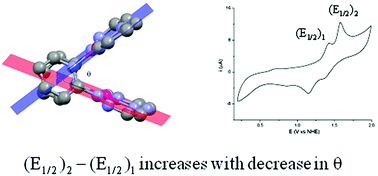
Condensing 2-hydrazinopyridine separately with glyoxal and benzil in 2 : 1 mole ratio, two osazones LH2 and L′H2·H2O (H: dissociable proton) are synthesized. Their reactions with K2[PdCl4] afford red [Pd(LH)]2(Cl)(ClO4) (1) and [Pd(L′H)]2(ClO4)2 (2). In the X-ray crystal structures, they contain [Pd2(ligand)2]2+ cations with ligand = LH or L′H. They are open clamshell type, with angles (θ) of 58.9° and 40.1° in 1 and 2 respectively. The respective Pd–Pd distances in 1 and 2 are found to be 3.2669(6) and 3.0128(5) Å. While this distance in 1 is almost equal to the sum of the van der Waals' radii of two Pd atoms, it is significantly less in 2. Theoretical calculations and structural factors indicate some sort of interaction between the two Pd(II) centers though the net bond order from the derived molecular orbitals for the Pd(II)2 core is found to be zero. The situation is similar to the crystal field splitting in tetrahedral copper(I) complexes. In 1 and 2, the interactions between the two Pd(II) centers increase as θ decreases. This is displayed in the difference in the redox potentials of the PdIIIPdII/PdIIPdII and PdIIIPdIII/PdIIIPdII couples in cyclic voltammetry, when compared with the same property in some similar dinuclear Pd(II) complexes known in the literature.


 Please wait while we load your content...
Please wait while we load your content...