Effects of the substituents of pyrazole/thiazine ligands on the magnetic properties of chloro-bridged Cu(ii) complexes†
Abstract
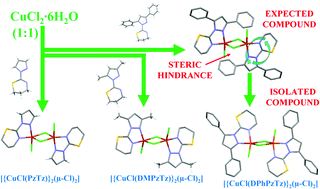
We have synthesised and characterised the dimeric copper(II) complexes [{CuCl(PzTz)}2(μ-Cl)2], [{CuCl(DMPzTz)}2(μ-Cl)2] and [{CuCl(DPhPzTz)}2(μ-Cl)2] and the monomeric complex [CuCl2(DMPzTz)] (PzTz = 2-(1-pyrazolyl)-1,3-thiazine, DMPzTz = 2-(3,5-dimethyl-1-pyrazolyl)-1,3-thiazine and DPhPzTz = 2-(3,5-diphenyl-1-pyrazolyl)-1,3-thiazine). Single crystal X-ray diffraction studies show that the geometry around the copper(II) center in the dimeric units is a distorted squared pyramid, while the monomeric compound presents a distorted squared planar coordination. The electronic and magnetic properties of complexes are discussed on the basis of their X-ray structures and EPR spectral studies combined with DFT calculations. Magnetostructural comparisons with structurally similar copper(II) complexes are also carried out. DFT calculations indicate that the dinuclear species are more stable than the mononuclear ones, although the inclusion of methyl or phenyl substituents provokes an important stabilization of the mononuclear forms. DFT calculations fail to predict the sign of the magnetic coupling constants of the complexes whereas multiconfigurational methods, CASSCF/NEVPT2 calculations, predict the correct sign of the exchange coupling constant.


 Please wait while we load your content...
Please wait while we load your content...