Adsorption of carbon dioxide on TEPA-modified TiO2/titanate composite nanorods†
Abstract
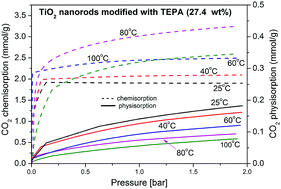
A titanate–TiO2 composite was obtained through hydrothermal treatment of TiO2 in KOH solution. The presence of a titanate phase was confirmed by X-ray diffraction (XRD), whereas scanning electron microscopy (SEM) measurements showed the porous nanorod structure of the material. The obtained nanorods were treated with tetraethylenepentamine (TEPA). Such synthesized sorbents were applied for CO2 removal. The CO2 capacity under a pressure of 1 bar and at 80 °C was 0.47, 0.34, and 3.11 mmol g−1 for the starting TiO2, the titanate–TiO2 composite and the TEPA–titanate–TiO2 composite (27.4 wt% of TEPA), respectively. The experimental isotherms of CO2 were analysed using the Langmuir, Freundlich, Sips, Toth, Unilan, Redlich–Peterson, Radke–Prausnitz, Dubinin–Radushkevich, Temkin and Pyzhev, and Jovanovich models. The error sums of squares (SSR) function was used to test the fit of the models. The analysis revealed that the Sips isotherm is the best-fitting model for the CO2 adsorption on the starting TiO2, whereas the Freundlich equation should be used to describe the CO2 adsorption isotherm on the titanate–TiO2 composite. The CO2 adsorption on the TEPA-modified sorbents was proposed to be described using the Sips isotherm for physical sorption and the modified Sips model for chemical sorption. The calculated isosteric heat of adsorption was found to be ≈46 kJ mol−1, which is about two times higher than the heat of CO2 absorption in liquid TEPA reported in the literature (i.e. ≈85 kJ mol−1). Therefore, it was concluded that the TEPA–titanate–TiO2 composite is an attractive alternative for liquid amines due to the lower energy of regeneration in the sorption–desorption process. The material was proved to be stable during multiple sorption–desorption cycles. Moreover, its thermal stability up to 150 °C was confirmed by thermogravimetric analysis (TGA). All these features make it a promising alternative for sorbents based on liquid amines.


 Please wait while we load your content...
Please wait while we load your content...