Microwave-assisted, ligand-free, direct C–H arylation of thiophenes in biomass-derived γ-valerolactone†
Abstract
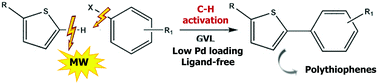
The Pd-catalyzed C–H arylation of heterocycles with aryl halides is a straightforward and more environmentally-friendly route to the synthesis of well-defined, pi-conjugated polymers for challenging applications in electronic devices. Although this type of transformation is more atom efficient than cross-couplings, it still poses environmental issues in the form of reaction media and the use of phosphine ligands. A procedure for the C–H arylation of thiophenes, with a substantially improved environmental impact, and a promising application of this protocol to the synthesis of regioregular polythiophenes are reported in this work. γ-Valerolactone (GVL), a non-toxic biomass-derived solvent, has been used in this phosphine-free microwave-assisted process and replaces commonly used dipolar aprotic media. Activated aryl bromides gave arylthiophenes in good yields, while iodides were used and pivalic acid was added when electron-withdrawing groups were present. The reaction was also extended to C–H (hetero)arylation polycondensation and a high molecular weight poly(3-hexyl)thiophene was obtained, using low Pd loading, in high yields and good regioregularity in short reaction times. Computational studies have shed light on GVL's role as a ligand in the catalytic system.


 Please wait while we load your content...
Please wait while we load your content...