A novel Pd(ii) CNO pincer complex of MR (methyl red): synthesis, crystal structure, interaction with human serum albumin (HSA) in vitro and molecular docking†
Abstract
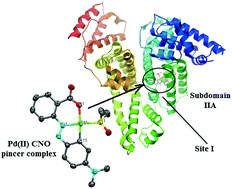
The C–H activation of methyl red (MR) (MR = 2-{[4-(dimethylamino)phenyl]diazenyl}benzoic acid) was achieved by reaction with Pd(OAc)2 under mild conditions. Metalation occurred at the ortho-position of the (dimethylamino)phenyl group in methyl red and the cyclometalated CNO pincer complex was obtained in good yield. The molecular structure shows that MR forms 6,5-fused chelate rings at the Pd(II) center. Interaction of the pincer complex with human serum albumin (HSA) has been studied by fluorescence spectroscopy and circular dichroism. Fluorescence quenching at different temperatures was analyzed using the classical Stern–Volmer equation, and a dynamic quenching mechanism was proposed. Energy resonance transfer between HSA and 1a′ was examined according to Förster's non-radiative energy transfer theory. A distance of about 5.06 Å between the complex and Trp214 (HSA) was obtained. Docking studies showed that the binding pocket of subdomain IIA of HSA is the preferable pincer complex binding site.


 Please wait while we load your content...
Please wait while we load your content...