Acyclonucleosides bearing coplanar arylethynyltriazole nucleobases: synthesis, structural analysis, and biological evaluation†
Abstract
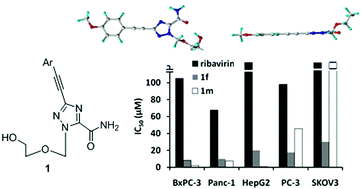
Nucleoside derivatives are an important class of molecules in the search for bioactive compounds. In our continuing efforts to develop novel nucleoside analogs, we used the Sonogashira cross-coupling reaction to synthesize 1,2,4-triazole acyclonucleosides bearing various arylethynyl groups on the triazole nucleobase. By employing 3-iodotriazole nucleoside as the coupling substrate, the Sonogashira reaction proceeded efficiently even with alkynes, which are notoriously unreactive and challenging. Further crystal structural analysis unveiled the coplanar feature of the 3-arylethynyltriazole motifs, suggesting their potential as surrogates for large planar aromatic systems or nucleobases. Most importantly, several synthesized compounds displayed interesting antiproliferative activity against various cancer cells and induced apoptosis, highlighting that this family of triazole nucleosides might show promise as anticancer candidates.


 Please wait while we load your content...
Please wait while we load your content...