The thermochemistry of long chain olefin isomers during hydroformylation†
Abstract
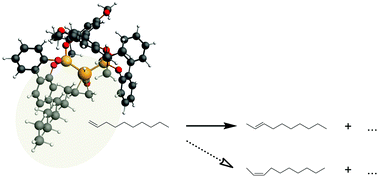
Rhodium-catalysed hydroformylation is the major industrial process to obtain aldehyde products from olefins. The isomerization of the olefin at the catalyst is the most prominent side reaction and lowers the overall yield of the process. We here investigate whether the olefin isomer distribution obtained from batch experiments using Rh(BiPhePhos) as a catalyst and n-decene as the olefin reaches the thermodynamic equilibrium and computational quantum chemical approaches are able to accurately reproduce the experimental olefin isomer distribution. The relative energies of cis/trans configurational and double bond positional isomers of long chain n-decene were calculated using Hartree–Fock, DFT and correlated ab initio methods. Results were compared to experimental data. Electron correlation was found to be critical for the description of cis-isomer relative energies. Dispersion corrections in the DFT calculations partially compensate for deficiencies and generally improve the agreement with experiment. Adding thermodynamic corrections suffers from the neglect of certain contributions to the entropy of flexible molecules. Accounting for the entropy of mixing of multiple conformers significantly reduces the deviation from experiment. The equilibrium distribution of long chain olefins is reasonably described by correlated QM and also some density functional methods. Computational thermochemistry has thus reached a state where it provides reliable parameters for complex reaction network models and process engineering.


 Please wait while we load your content...
Please wait while we load your content...