Complexes and salts of the nitrogen-rich triazole–tetrazole hybrid ligand with alkali and alkaline earth metal cations: experimental and theoretical findings†
Abstract
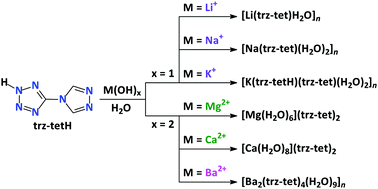
Reaction of the nitrogen-rich triazole–tetrazole hybrid ligand 5-(4H-1,2,4-triazol-yl)-2H-tetrazole (trz–tetH) with Li+, Na+, K+ and Ba2+ in water leads to the coordination polymers [Li(trz–tet)H2O]n, [Na(trz–tet)(H2O)2]n, [K(trz–tetH)(trz–tet)(H2O)2]n and [Ba2(trz–tet)4(H2O)9]n, exhibiting different topologies and coordination modes. Salt-like structures with anionic triazole–tetrazole building blocks [Mg(H2O)6](trz–tet)2 and [Ca(H2O)8](trz–tet)2 were also obtained. All structures were simplified by topological analysis, which showed that the structures of [Mg(H2O)6](trz–tet)2 and [Ca(H2O)8](trz–tet)2 can be considered as underlying networks, constructed from the hydrogen bonded [Mg(H2O)6]2+ or [Ca(H2O)8]2+ cations and trz–tet ligands, with the rare binodal 5,10-connected topology alb-5,10,P21/c-1 and a unique binodal 5,12-connected topology, respectively. According to TGA/DTA analysis, all compounds show endothermic mass losses below 200 °C due to dehydration processes. The dehydrated residues are stable up to 300 °C. It was also established that the anionic form of the coordinated ligand trz–tet decomposes with an abrupt mass loss accompanied by a sharp and intense exothermic effect, while the non-coordinated trz–tet, as in the structures of the Mg- and Ca-based compounds, decomposes with a more gradual mass loss and significantly broad exothermic effect. Static DFT, ab initio molecular dynamics simulations as well as charge and energy decomposition (ETS-NOCV) based studies are performed in order to shed light on the stability of the newly obtained crystals.


 Please wait while we load your content...
Please wait while we load your content...