Facile and green one pot synthesis of zinc sulphide quantum dots employing zinc-based ionic liquids and their photocatalytic activity†
Abstract
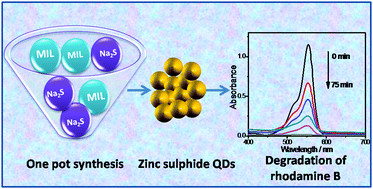
Herein, an economical and green method for the preparation of zinc sulphide (ZnS) quantum dots is presented, in which the zinc (metal M) containing ionic liquid (MIL) [Cnmim][ZnCl3] (n = 4, 8, and 16) is used as a precursor, template and solvent. The ZnS quantum dots (QDs) are synthesized at room temperature via a simple grinding method. The prepared QDs are characterized via X-ray diffraction (XRD), transmission electron microscopy (TEM), UV-Vis spectroscopy, and photoluminescence spectroscopy (PL). The XRD pattern of the investigated QDs suggests a zinc blende structure, where broadening of the diffraction peaks with an increase in the alkyl chain length of the MILs is observed. The ZnS QDs are found to be spherical in shape with an average diameter of 1.5–5.0 nm, which varies with the length of the MIL alkyl chain. The application of the prepared ZnS QDs towards the photocatalytic degradation of Rhodamine B under UV-light has also been investigated and discussed. The size and optical and photocatalytic properties of the ZnS QDs are found to be dependent upon the length of the MIL alkyl chain. The simple and economical method presented for the preparation of QDs and their MIL-dependent characteristics provide a new approach to manipulate properties at the nanoscale.


 Please wait while we load your content...
Please wait while we load your content...