Sulfonamide vs. sulfonimide: tautomerism and electronic structure analysis of N-heterocyclic arenesulfonamides†
Abstract
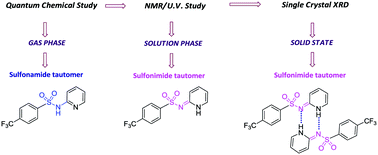
A systematic study of the structure and electronic properties of N-heterocyclic arenesulfonamides (NHAS) was performed using experimental and theoretical methods. Ten new examples of NHAS with pyridine and thiazole N-heterocycles were synthesized. X-ray diffraction studies on one of the compounds suggested that the sulfonimide form is preferred; the molecule exists as a dimer due to two strong intermolecular hydrogen bonds. The possibility of sulfonamide (N-sulfonylamine) ![[leftrightharpoons]](https://www.rsc.org/images/entities/char_21cb.gif) sulfonimide (N-sulfonylimine) tautomerism was explored using density functional theory (DFT) methods. The energy difference between the two tautomers was small (<6 kcal mol−1) in the gas phase, favoring the sulfonamide tautomer; however, with an increase in the polarity of the solvent, the preference towards the sulfonimide tautomers increased in many examples. Electronic structural analysis of the sulfonimide tautomers suggested the possibility of an unusual (NHC) → N coordination.
sulfonimide (N-sulfonylimine) tautomerism was explored using density functional theory (DFT) methods. The energy difference between the two tautomers was small (<6 kcal mol−1) in the gas phase, favoring the sulfonamide tautomer; however, with an increase in the polarity of the solvent, the preference towards the sulfonimide tautomers increased in many examples. Electronic structural analysis of the sulfonimide tautomers suggested the possibility of an unusual (NHC) → N coordination.


 Please wait while we load your content...
Please wait while we load your content...