A TDDFT study on the excited-state double proton transfer reaction of 8-hydroxyquinoline along a hydrogen-bonded bridge
Abstract
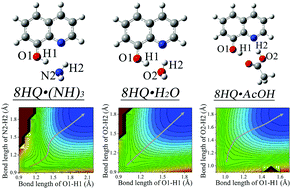
The mechanism of the excited-state double proton transfer (ESDPT) reactions of 8-hydroxyquinoline (8HQ) along three types of hydrogen-bonded bridges—ammonia (NH3), water (H2O) and acetic acid (AcOH) has been investigated by using time-dependent density functional theory. Based on the analysis of hydrogen bond strengths and the excited-state potential energy surfaces (PES) along the proton transfer coordinates, it is concluded that the hydrogen bonds play the key role in the excited-state multiple proton transfer reaction. Moreover, three different concerted mechanisms have been found in 8HQ·NH3, 8HQ·H2O and 8HQ·AcOH complexes. Upon photoexcitation, the deprotonation of a hydroxyl group in 8HQ would occur first in 8HQ·NH3 due to the stronger hydrogen bond –OH⋯NH3. On the contrary, the hydrogen bond –OH⋯O–C–OH is much weaker in the 8HQ·AcOH complex, and this leads to a fast protonation of –N– in the 8HQ moiety. For the 8HQ·H2O complex, the hydrogen bond strengths are almost the same, so that both protons would transfer simultaneously in a symmetrical and concerted fashion.


 Please wait while we load your content...
Please wait while we load your content...