Structural analysis and magnetic induced hyperthermia of Fe3+ and Mn2+ substituted β-Ca3(PO4)2
Abstract
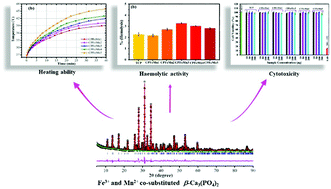
The design of attractive materials for application in hyperthermia therapy has been in huge demand during recent years. To achieve this aim, magnetic features are induced in bioactive β-tricalcium phosphate [β-TCP, β-Ca3(PO4)2] through Fe3+ and Mn2+ co-substitution. A series of Fe3+/Mn2+ co-substitutions in β-Ca3(PO4)2 are synthesized through a co-precipitation route. Detailed structural analysis emphasizes the formation of β-Ca3(PO4)2 solid solutions with Fe3+/Mn2+ co-substitutions. Both Fe3+ and Mn2+ prefer to occupy the Ca2+(5) site of β-Ca3(PO4)2 up to a limit of ∼10 mol% and thereafter get accommodated at the Ca2+(4) lattice site. The synthesized materials exhibit magnetic features and heating ability tests certify their potential role in hyperthermia applications. Haemolytic and cytotoxicity tests confirm the biocompatibility of the synthesized thermoseeds.


 Please wait while we load your content...
Please wait while we load your content...