Construing the interactions between MnO2 nanoparticle and bovine serum albumin: insight into the structure and stability of a protein–nanoparticle complex†
Abstract
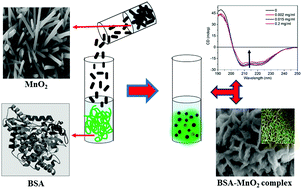
A systematic study of the interaction of bovine serum albumin (BSA) with MnO2 nanoparticles (NPs) was carried out. MnO2 nanoparticles were prepared via a low temperature (90 °C) single-step precipitation route in the absence of surfactant/template and characterized using XRD, TEM, FESEM, FTIR, XPS, and Raman spectroscopy. MnO2 particles were found to have a length of 900 nm and a diameter of 10 nm. The interaction of BSA with NP was investigated using various spectroscopic and biophysical techniques under physiological pH 7.4. MnO2 effectively quenches the intrinsic fluorescence of BSA through static quenching. The effect of MnO2 on the conformation of BSA was analyzed using circular dichroism (CD), Fourier transform infrared (FTIR), and Raman spectroscopy, and it was observed that the secondary structure of BSA altered after the interaction with MnO2. Thermal CD spectroscopy revealed insignificant variation in the melting temperature of BSA upon binding to MnO2. Additionally, the morphological changes of BSA upon interaction with MnO2 were characterized using a field emission scanning electron microscope (FESEM). Moreover, the increase in the size of the BSA–MnO2 complexes was analyzed using dynamic light scattering (DLS). This study shows that the adsorption of BSA on MnO2 is dependent on the concentration of the protein as well as the NP.


 Please wait while we load your content...
Please wait while we load your content...