Decoration of Pd and Pt nanoparticles on a carbon nitride (C3N4) surface for nitro-compounds reduction and hydrogen evolution reaction†
Abstract
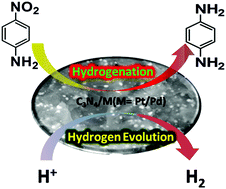
Herein, we propose the synthesis of Pd and Pt monometallic nanoparticles on a carbon nitride (C3N4) surface for the reduction of nitro compounds as well as for electrocatalysis. For the synthesis of C3N4/Pd and C3N4/Pt, metal ions were initially adsorbed on the C3N4 surface and then subsequently reduced by NaBH4. The as-synthesized heterostructures were authenticated by different characterization techniques: UV-vis, PXRD, XPS, TEM, FESEM, and EDS. Decorations of monometallic NPs on C3N4 not only improved the reduction efficiency of nitro-compounds but also enhanced the electrocatalytic activity in the hydrogen evolution reaction. C3N4/Pt proved to be an efficient electrocatalyst as it requires a potential of −0.339 V to attain a current density of 10 mA cm−2; whereas, C3N4/Pd requires −0.371 V to reach a current density of 10 mA cm−2vs. Ag/AgCl. Both C3N4/Pd and Pt heterostructures are better than bare C3N4, which needs −0.596 V to achieve a current density of 10 mA cm−2vs. Ag/AgCl. On the other hand, C3N4/Pd showed a better performance in nitro-compound reduction compared to C3N4/Pt and bare C3N4. The kinetic study reveals that the rate constant using a C3N4/Pd catalyst is 6.7 × 10−1 min−1 for p-nitroaniline reduction, which is 101 times higher compared to bare C3N4 and 4.7 times higher in comparison to C3N4/Pt.


 Please wait while we load your content...
Please wait while we load your content...