A trigonal prismatic anionic iron(iii) complex of a radical o-iminobenzosemiquinonate derivative: structural and spectral analyses†
Abstract
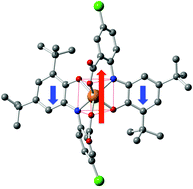
A new iron(III) complex, [Et3NH][FeIII(L2−˙)2] (1) with a substituted o-aminophenol based ligand is reported. Complex 1 is an anionic complex with a triethylammonium cation in the lattice. It contains two O,O,N-coordinated o-iminobenzosemiquinonate(2−) radical anions with an Fe(III) centre in a high-spin configuration. The crystal structure of 1 was determined by X-ray diffraction, which revealed a trigonal prismatic coordination environment whose electronic structure was established by various physical methods including EPR, Mössbauer spectroscopy and variable-temperature (2–300 K) magnetic susceptibility measurements. Electrochemical analysis indicated primarily ligand-centred redox processes. Complex 1 retained the high-spin character of Fe(III) throughout the temperature range studied (2–300 K) and exhibited, as expected, strong antiferromagnetic coupling operating between two radicals (SR = 1/2) and the high-spin Fe(III) centre (SFe = 5/2), yielding a St = 3/2 as the ground state. This was confirmed by means of DFT calculations (M06-2X/6-31+G*) and a spin density analysis of the high spin and low spin configurations.


 Please wait while we load your content...
Please wait while we load your content...