Solvent-dependent gelation behaviour and liquid crystal properties of a bent-core dihydrazide derivative†
Abstract
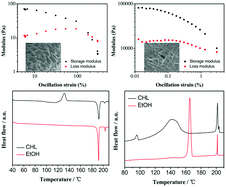
Herein, a bent-core dihydrazide derivative (BP8-C) showed unusual solvent-induced optional self-assembly behaviour, e.g. crystalline gels obtained from ethanol (EtOH) and liquid crystalline gels obtained from chloroform (CHL) and toluene (Tol). Packing models in crystalline and liquid crystalline states are confirmed by the XRD method. It was demonstrated by fluorescence spectroscopy that gelators aggregate in EtOH in a way n = 2.13 (Avrami parameter). Interestingly, liquid crystalline gels obtained in CHL display better elastic property with 200% cross-over strain and act as Newtonian fluid at low shearing rate regions, whereas the crystalline gels show better viscidity with low cross-over strain (3%). Moreover, BP8-C xerogels obtained from CHL exhibit Tg transition followed by cold crystallization upon the first heating run, whereas an unknown mesophase, Colh phase, and a broadened and super-cooled crystallization were observed during cooling. In contrast, xerogels obtained from EtOH show enantiotropic Colh mesophase, and sharp crystallization transition. The different gelation behaviour and the liquid crystalline properties of the xerogels obtained from CHL and ethanol were attributed to different molecular aggregates due to interaction between ethanol and the gelators, as confirmed by 1H NMR and FTIR spectroscopy. We emphasize the role of solvent on the self-assembly degree of gelators, which can further influence the properties of gels and the corresponding xerogels.


 Please wait while we load your content...
Please wait while we load your content...