Synthesis and oxidative stability of hyperbranched macromolecule-bridged hindered phenols
Abstract
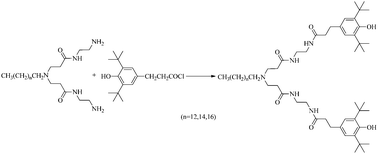
A series of hyperbranched macromolecule-bridged hindered phenols was successfully synthesized with 3-(3,5-di-tert-butyl-4-hydroxy-phenyl)-propionyl chloride and a series of 1.0 generation (1.0G) hyperbranched macromolecules. Their antioxidant ability was investigated by DPPH˙ scavenging assay and oxygen uptake test to establish the correlation between the alkyl-chain length of the core in the bridged group and antioxidant property of hyperbranched macromolecule-bridged hindered phenols. Effective concentration that scavenges 50% radical (EC50), reaction rate that scavenges 50% radical (TC50) and antiradical efficiency (AE) have been reported with DPPH˙ assay, and the results indicate that the antioxidant activity decreased with an increase in the alkyl-chain length of the bridged groups. The inhibition period (tinh), the rate constant of inhibition (kinh) and the stoichiometric factor (n) were used to evaluate the ability of inhibiting styrene against AIBN-induced oxidation for hyperbranched macromolecule-bridged hindered phenols. The results showed that the steric effect of the hyperbranched macromolecule-bridged hindered phenols increased with an increase in the alkyl-chain length of the bridged groups, which led to a decline in the reactivity between the hindered phenols and free radicals produced in the AIBN-induced oxidation process of styrene. For the simple free radical systems, the oxidative stabilities of the hyperbranched macromolecule-bridged hindered phenols decreased with an increase in the alkyl-chain length of the bridged groups.


 Please wait while we load your content...
Please wait while we load your content...