Polymorphism and benzene solvent controlled stimuli responsive reversible fluorescence switching in triphenylphosphoniumfluorenylide crystals†
Abstract
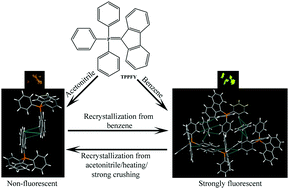
Triphenylphosphoniumfluorenylide (TPPFY), a fluorescent fluorene attached molecule, showed polymorphism and benzene solvent induced aggregation enhanced emission (AEE) in the solid state. Crystallization from CH3CN produced non-fluorescent crystals of TPPFY (TPPFY-1), whereas intense yellow fluorescent crystals (plates and blocks) were obtained from benzene (TPPFY-2, λmax = 538 nm, Φf = 38%). Structural analysis indicates that TPPFY-1 exhibits strong π⋯π interactions (3.371–3.399 Å) between fluorene units in the crystal lattice that quenched the solid state fluorescence. In contrast, the inclusion of a benzene molecule in TPPFY-2 prevents the close packing of fluorophores and shows intense yellow fluorescence in the solid state. TPPFY-2 showed only weak C–H⋯π interactions between fluorene and the phenyl group of triphenylphosphoniumylide. Hirshfeld surface analysis further supported the differences in the intermolecular interactions and molecular packing between TPPFY-1 and TPPFY-2. Interestingly, heating/strong crushing irreversibly converts fluorescent TPPFY-2 crystals to non-fluorescent TPPFY-1 crystals. However, recrystallization of TPPFY-1 from benzene produced fluorescent TPPFY-2 crystals. Thus TPPFY displays reversible off–on fluorescence switching heating/crushing and recrystallization. PXRD studies confirmed the polymorphism as well as the phase conversion of TPPFY-2 to TPPFY-1 by external stimuli. This study indicates the role of the benzene molecule in controlling the molecular packing and functional properties of TPPFY.


 Please wait while we load your content...
Please wait while we load your content...