Structures of dimetallocenes M2(C5H5)2 (M = Zn, Cu, Ni, Co, Fe) and their perfluorinated derivatives†
Abstract
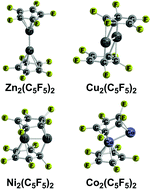
The unexpected 2004 discovery of decamethyldizincocene suggested some new possibilities for organometallic compounds in which an M2 unit is sandwiched between two planar carbocyclic rings. Density functional theory shows that the low-energy structures for the dizincocenes Zn2(C5X5)2 (X = H, F) are singlet coaxial structures having two (η5-C5X5)Zn units linked by a Zn–Zn single bond of length ∼2.3 Å. However, the low-energy M2(C5H5)2 (M = Cu, Ni, Co, Fe) structures have perpendicular configurations with bridging C5H5 ligands. They exhibit increasingly higher spin states from singlet (Cu, Ni) to triplet (Ni,Co), quintet (Co,Fe), and septet (Fe). The low-energy structures of the perfluorinated systems M2(C5F5)2 (M = Co, Fe) have irregular geometries with one bent bridging C5F5 ring and one planar terminal pentahapto η5-C5F5 ring, with the metal atom bearing the terminal η5-C5F5 ligand has the favored 18-electron configuration while the other metal atom has only a lower electron configuration and vacant coordination sites.


 Please wait while we load your content...
Please wait while we load your content...