Fullerene C60 dianion salt, (Me4N+)2(C602−)·(TPC)2·2C6H4Cl2, where TPC is triptycene, obtained by a multicomponent approach†
Abstract
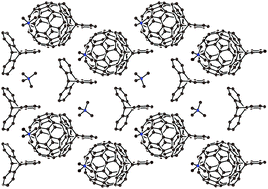
A multicomponent approach provided a new salt (Me4N+)2(C602−)·(TPC)2·2C6H4Cl2 (1) containing the discrete C602− dianions, where TPC is triptycene. The crystal structure consists of alternating layers of closely packed zigzag fullerene chains solvated with C6H4Cl2 molecules and the TPC network with Me4N+ cations. The hexagonal vacancies in the TPC network accommodate the cations and simultaneously act as a template for the formation of zigzag fullerene chains. Optical spectra support the formation of C602− dianions. According to the electron paramagnetic resonance technique (EPR) the C602− dianions have a diamagnetic singlet ground state below 140 K. The increased intensity of the EPR signal above this temperature reflects the growing thermal population of the excited triplet state. This signal has g = 2.0003 and a linewidth of 2.53 mT at 302 K and the estimated singlet–triplet energy gap is 674 ± 10 K (468 ± 7 cm−1).


 Please wait while we load your content...
Please wait while we load your content...