Efficient synthetic route to aromatic secondary amines via Pd/RuPhos/TBAB-catalyzed cross coupling†
Abstract
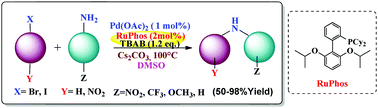
Herein, C–N cross coupling methodology was developed for the synthesis of a diverse range of nitro-substituted secondary amines. A variety of strained, aliphatic, and aromatic precursors were effectively used, with low catalyst and ligand loading ratios resulting in product formation in good yield. This method can act as an alternative to nucleophilic addition reactions. To cross couple electron-donating, electron-withdrawing, neutral, and aliphatic primary amines with alkyl/aryl halides, a combination of RuPhos and TBAB was carefully tuned. Further, characterization of these molecules was carried out using FT-IR, 1H-NMR, 13C-NMR, 19F-NMR, single crystal XRD, and C, H, and N elemental analyses.


 Please wait while we load your content...
Please wait while we load your content...