Epoxidation of ethylene over Pt-, Pd- and Ni-doped graphene in the presence of N2O as an oxidant: a comparative DFT study†
Abstract
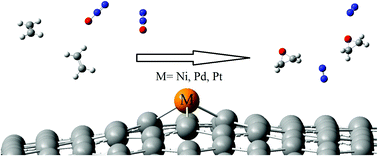
Ethylene oxide (EO) is an important intermediate for the synthesis of fine chemicals. Therefore, the oxidation of ethylene to EO is an industrially important reaction. The catalytic oxidation of ethylene to EO by N2O on Pt-, Pd- and Ni-doped graphene nanosheets have been probed theoretically using the M06-2X hybrid DFT method. DFT calculations suggest that catalytic oxidation proceeds in three steps. The reaction starts with the decomposition of N2O to form activated atomic oxygen (Oads), which is adsorbed on the surface, and N2 molecule. Then, an ethyleneoxy intermediate is produced from the addition of ethylene to Oads. Finally, the cyclization of ethyleneoxy intermediate results in EO as a desirable product. Our findings demonstrate that compared to Pt- or Pd-doped graphene, the Ni-doped surface is an efficient catalyst for the epoxidation of ethylene to EO by N2O under mild conditions. The catalytic epoxidation method demonstrated herein is a green approach, as N2 gas is the only by-product, at the expense of the greenhouse gas-N2O.


 Please wait while we load your content...
Please wait while we load your content...