White light emission from fluorene-EDOT and phenothiazine-hydroquinone based D–π–A conjugated systems in solution, gel and film forms†
Abstract
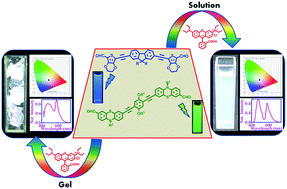
Two conjugated D–π–A organic molecules, one fluorene-ethylene dioxythiophene (FL-E) based and another phenothiazine-hydroquinone (PT-Hq) based, were synthesized and observed to form 1-dimensional microstructures in the solid state. These two molecules when mixed with rhodamine B dye (Rh-B) and photoexcited at 411 nm generated white light with Commission Internationale d'Eclairage (CIE) coordinates (0.32, 0.33) in the solution state. Similarly, white light was also obtained in the poly(methyl methacrylate) film (0.30, 0.33) and in the gelatine gel (0.31, 0.34) for the same composition of the mixture as in the solution state. The correlated colour temperatures obtained in the three phases (6111 K in solution, 6900 K in the PMMA film and 6400 K in the gelatin gel) suggest that the system emits cool white light. In order to verify the D–π–A nature of the molecules, intramolecular charge transfer studies were conducted, which revealed that both the molecules showed positive solvatochromism. The plausible mechanism behind white light emission is Forster resonance energy transfer accompanied by simultaneous emission. This was verified through fluorescence titration experiments, lifetime studies, spectral overlap and Forster distance calculations and correlated with the results obtained from cyclic voltammetry and DFT studies. This is the first report on the emission of white light from the D–π–A system by FRET.


 Please wait while we load your content...
Please wait while we load your content...