Square planar Ni(ii) complexes of acetone N4-phenyl-thiosemicarbazone and in situ generated benzoyl thiosemicarbazide ligands: synthesis, spectral and structural characterizations, thermal behaviour and electrochemical studies†
Abstract
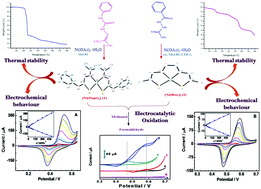
Two novel square planar complexes [Ni(Hapt)2] (1) and [Ni(btsc)] (2) have been synthesized from acetone N4-phenyl-thiosemicarbazone (Hapt) and benzoyl-3-thiosemicarbazide (Hbtsc) by the reaction of nickel(II) salt. The ligand and complexes have been characterized by various physicochemical methods. Complexes 1 and 2 crystallize in the monoclinic and orthorhombic systems with space group C2/c and Pbcn, respectively. In complexes 1 and 2 the nickel centre is coordinated through the nitrogen and sulphur atoms forming a square planar geometry. Complexes 1 and 2 are stabilized via various types of intermolecular interactions. The course of the thermal degradations of complexes 1 and 2 has been investigated by TGA which indicated that a metal sulphide/oxide is formed as the final residue. Cyclic voltammetric studies show that complex 1 exhibits a reversible Ni(II)/Ni(III) redox process at 0.525 V (E1/2 value at 20 mV s−1) while complex 2 shows reversible redox behavior with an E1/2 value of 0.43 V. Furthermore, efficient electrocatalytic properties of complexes 1 and 2 toward the oxidation of hydrazine and methanol are demonstrated after immobilizing the complexes into Nafion films. The electrocatalytic properties may find applications in industry, in methanol sensing and in methanol fuel cells.


 Please wait while we load your content...
Please wait while we load your content...