In operando X-ray diffraction of lithium–oxygen batteries using an ionic liquid as an electrolyte co-solvent
Abstract
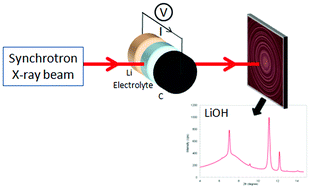
A real-time synchrotron X-ray diffraction technique is applied to study the oxidation/reduction of lithium oxide derivatives in an operating lithium–air battery cell containing a Room Temperature Ionic Liquid (RTIL) in its electrolyte. Four different electrolytes are tested, composed of dimethylsulfoxide (DMSO), a RTIL and lithium perchlorate salt (LiClO4). This study proves that lithium as an anode material reacts with the RTIL, forming lithium hydroxide (LiOH) spontaneously, independently of the cycling stage. Comparisons between different electrolytes provide insights for future investigation of the improvement of electrolyte design of this technology.


 Please wait while we load your content...
Please wait while we load your content...