α,α-Difluoro-β-iminophosphonates, an alternative strategy towards the synthesis of α,α-difluoro-β-aminophosphonate derivatives†
Abstract
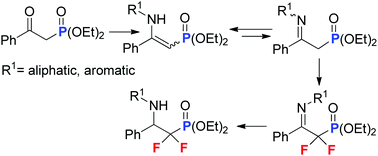
α,α-Difluoromethylenephosphonates are generally known as stable natural phosphate mimics with significantly improved biological activities. A number of α,α-difluoroalkylphosphonates have already been found to be important enzyme inhibitors. Herein, we report an alternative strategy towards the synthesis of α,α-difluoromethylene-β-aminophosphonate derivatives. A small library of N-substituted α,α-difluoro-β-aminophosphonates was designed and described via NMR study. The protocol began with the condensation of β-ketophosphonates with a series of primary amines. The key step of the synthesis is an electrophilic fluorination, mediated by Selectfluor®, of the mixture of β-enamino/β-iminophosphonates leading to α,α-difluoro-β-iminophosphonates followed by reduction. The title compounds may behave as convenient building blocks for further more advanced modification.


 Please wait while we load your content...
Please wait while we load your content...