Condensation mechanism of cage hexabenzylhexaazaisowurtzitane from glyoxal and benzylamine: a computational study†
Abstract
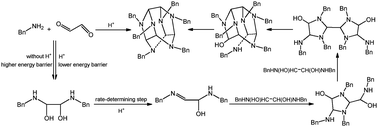
A computational study was performed on the condensation mechanism for the formation of cage hexabenzylhexaazaisowurtzitane (HBIW) from glyoxal and benzylamine. The results suggest that the intermediate 1,2-bis(benzylamino)-1,2-ethanediol is present in the reaction system, especially in the presence of acid catalyst. However, it is unfavorable for N,N′-dibenzyl-1,2-ethanediimine to be formed without the use of acid because the energy barriers for elimination of two water molecules from 1,2-bis(benzylamino)-1,2-ethanediol are 51.99 kcal mol−1 and 60.49 kcal mol−1. The acid-catalyzed water elimination reaction of 1,2-bis(benzylamino)-1,2-ethanediol decreases to 17.17 kcal mol−1, resulting in the formation of another intermediate of 1-(benzylamino)-2-(benzylimino)-ethanol. The 1-(benzylamino)-2-(benzylimino)-ethanol reacts with another 1,2-bis(benzylamino)-1,2-ethanediol to provide HBIW through four cyclization reactions.


 Please wait while we load your content...
Please wait while we load your content...