Conversion of hydrazides into N,N′-diacylhydrazines in the presence of a ruthenium(ii)–arene complex†
Abstract
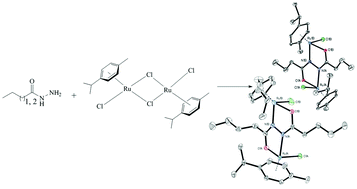
Mono and dinuclear p-cymene-ruthenium(II) complexes [RuCl(L1)(η6-p-cymene)]Cl, where L1 is propionic acid hydrazide (1) and [Ru2Cl2(L2)(η6-p-cymene)2], where H2L2 is N1,N2-dipropionylhydrazine (2), were prepared by a reaction of [RuCl2(η6-p-cymene)]2 with the corresponding ligand precursor. Upon the reaction of [RuCl2(η6-p-cymene)]2 with butyric acid hydrazide and pentanoic acid hydrazide in a 1 : 1 molar ratio in situ formation of tetradentate bridging ligands, N1,N2-dibutanoylhydrazine and N1,N2-dipentanoylhydrazine, respectively, occurred and the dinuclear complexes [Ru2Cl2(L3)(η6-p-cymene)2] (3) and [Ru2Cl2(L4)(η6-p-cymene)2] (4) were isolated. The compounds were characterised by elemental analysis, ESI-mass spectrometry, IR and 1D and 2D NMR spectroscopies. The structures of all complexes were established using single crystal X-ray crystallography. According to these data in both the mono- and dinuclear complexes the ruthenium atoms adopt the usual “three-leg piano-stool” geometry which is common for this type of complexes. Combining DFT calculations with the characterisation of the final products using X-ray diffraction, a possible reaction mechanism was discussed.


 Please wait while we load your content...
Please wait while we load your content...