Investigation on the effect of an anion layer on photocatalytic activity: carbonate vs. oxalate†
Abstract
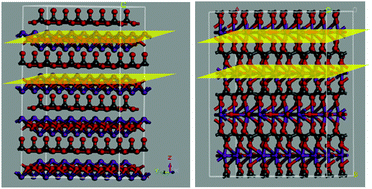
In a layer structure, the influence of an anion layer on the photochemical property is still unknown. In this study, we mainly investigated the photochemical properties of Bi2O2CO3 and Bi(C2O4)OH. It has been found that although they have similar layer structures, Bi2O2CO3 shows activities 1.15 and 2.48 times higher than those of Bi(C2O4)OH for the degradation of phenol and rhodamine B (RhB) under UV light irradiation (λ ≤ 400 nm), respectively; these high activities have been mainly attributed to their different anion layers. For Bi2O2CO3, CO32− ions connect with one another to form a linked anion layer, whereas the alternating [Bi2O2]2+ and CO32− layers are completely separate; however, for Bi(C2O4)OH, C2O42− ions do not link with each other, whereas oxygen bridges are formed between the [Bi2O2]2+ and C2O42− layers. The results demonstrate that an internal electric field (IEF) is favored by the separate [Bi2O2]2+ and CO32− layers, which can greatly improve the charge separation rate. In contrast, the oxygen bridges between the [Bi2O2]2+ and C2O42− layers do not favor the formation of an IEF, resulting in a low charge separation rate. This finding suggests that layer semiconductors with completely separate cation and anion layers can be developed as efficient photocatalysts.


 Please wait while we load your content...
Please wait while we load your content...