A new fluorescent sensor containing glutamic acid for Fe3+ and its resulting complex as a secondary sensor for PPi in purely aqueous solution†
Abstract
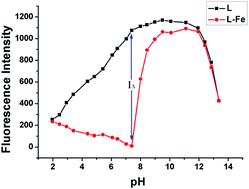
A new fluorescence sensor L with benzimidazo[2,1-α]benz[de]isoquinoline-7-one as the fluorophore and glutamic acid moiety as the receptor was synthesized and characterized. In 100% aqueous buffer (tris 10 mM, pH = 7.4) solution, L showed complete fluorescence quenching in the presence of Fe3+ over other competitive metal ions. It worked based on the Fe3+-induced formation of a 1 : 1 L–Fe3+ complex, producing a reverse-PET mechanism effect. The quantum yield was determined to be 0.28 and 0.04 for free L and L–Fe3+ complexes, respectively. The L–Fe3+ ensemble could serve as a subsequent sensor for PPi due to the strong attraction between Fe3+ and PPi by fluorescence recovery without the interference of the biological competitors including ATP, ADP and PO43−. The LOD calculated by 3σ/s methods was found to be as low as 8 × 10−7 M for Fe3+ and 1.53 × 10−6 M for PPi. The DFT and a solvent-dependent (H2O) TDDFT calculations also confirmed the proposed reverse-PET mechanism indirectly.


 Please wait while we load your content...
Please wait while we load your content...