A theoretical elucidation: why does a SO3H-functionalized imidazolium-based ionic liquid catalyze the conversion of 5-hydroxymethylfurfural to levulinic acid?†
Abstract
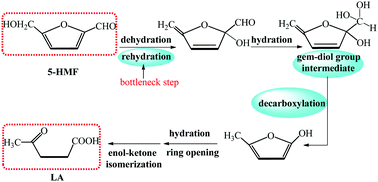
This work presents a density functional theory (DFT) study on the molecular mechanism for conversion from 5-hydroxymethylfurfural (HMF) to levulinic acid (LA) catalyzed by a representative of SO3H-functionalized imidazolium-based ionic liquids (ILs), 1-methyl-3-(3-sulfopropyl)-imidazolium hydrogen sulfate ([C3SO3Hmim]HSO4). The calculated overall barrier of the catalytic conversion is 34.7 kcal mol−1, which is compatible with the efficient transformation of 5-HMF into LA in the IL under mild heating conditions. The present calculations emphasize the dependence of the catalytic performance of the IL on the acidity and nucleophilicity of its constituent ions.


 Please wait while we load your content...
Please wait while we load your content...