Pd(NHC)PEPPSI-diazonium salts: an efficient blend for the decarboxylative Sonogashira cross coupling reaction†
Abstract
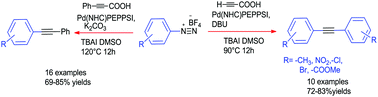
An efficient N-heterocyclic carbene based Pd complex is described for the de-carboxylative Sonogashira coupling reaction with diazonium salts under ligand and co-catalyst free conditions. Pd(NHC)PEPPSI not only offers an air and moisture stable pre-catalyst but also efficiently catalyzes the reaction with lower loading. Various aryl and hetero aryl diazonium salts undergo an sp–sp2 cross coupling reaction with alkynyl acid to give good to excellent yields of substituted acetylene derivatives. The diazonium salts serve as efficient, easily available, and inexpensive aryl surrogates over conventional aryl halides, triflates, oxalate, and sulphamate.


 Please wait while we load your content...
Please wait while we load your content...