Synthesis and characterization of three pyrazolate inner diazonium salts: green, powerful and stable primary explosives†
Abstract
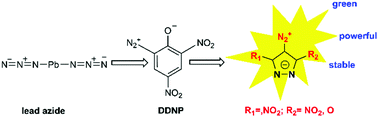
The synthesis and energetic performances of three inner diazonium salts, 3,5-dichloro-4-diazopyrazole zwitterion (1), 4-diazo-3,5-dinitropyrazole zwitterion (2), and 4-diazo-5-nitro-pyrazol-3-one zwitterion (3), were investigated in this study. All these compounds were characterized by IR, UV/Vis, 13C and 15N NMR spectroscopy, and elemental analysis. Their structures were further confirmed by single crystal X-ray diffraction. Moreover, their thermal stabilities are determined by differential scanning calorimetry (DSC). In addition, detonation parameters (e.g. detonation velocity and pressure) of the target compounds were computed using EXPLO5 v6.01 based on the calculated heat of formation and density. The results show that compound 2 exhibits a density of 1.849 g cm−3 and a decomposition temperature (Td) of 154 °C, which are superior to those of the efficient primary explosive DDNP (2-diazo-4,6-dinitrophenol, Td = 142 °C). Compounds 1 and 3 also have moderately high decomposition temperatures of 135 and 151 °C, respectively. Besides, compounds 2 and 3 exhibit good detonation properties (2, 9038 m s−1, 35.0 GPa; 3, 8055 m s−1, 26.4 GPa), which are higher than those of the widely used primary explosives DDNP (7290 m s−1, 23.7 GPa) and Pb(N3)2 (5876 m s−1, 33.4 GPa). The moderately high thermal stabilities combined with the good detonation properties make them potential green primary explosives.


 Please wait while we load your content...
Please wait while we load your content...