Photorelease of nitric oxide (NO) on ruthenium nitrosyl complexes with phenyl substituted terpyridines†
Abstract
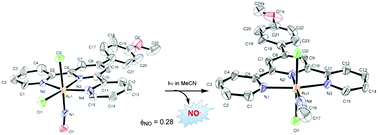
We investigated the influence of the nature of the substituent on the 4′-position of terpyridine ligands and the geometry around the metal ion on the photosensitization of ruthenium nitrosyl complexes. This was achieved by synthesizing a series of [Ru(R-Phtpy)Cl2(NO)]+ complexes based on tridentate terpyridine ligands with a substituted phenyl ring on the central pyridine (R = NO2, H, Br, OMe). The properties of both cis-(Cl,Cl)[Ru(R-Phtpy)Cl2(NO)]+ and trans-(Cl,Cl)[Ru(R-Phtpy)Cl2(NO)]+ isomers are presented. The optimized geometries and UV-Vis spectra were fully computed by DFT calculations and are in agreement with the experimental data. Quantum yields of NO release were obtained by numerical modeling and parameter optimization on the time evolution of the electronic spectra during irradiation. On irradiation with visible light in MeCN both cis(Cl,Cl)- and trans(Cl,Cl)-[Ru(MeO-Phtpy)Cl2(NO)](PF6) undergo facile photorelease of NO with the concomitant formation of the photoproduct as the solvate. Unambiguous characterization of the photoproduct for R = OMe was achieved by X-ray analysis and revealed that both isomers lead to trans(Cl,Cl)-[Ru(MeO-Phtpy)Cl2(MeCN)](PF6) as the unique photoproduct. The calculation of the relaxed potential energy surface confirmed an internal rearrangement of the cis(Cl,Cl) isomer to the trans(Cl,Cl) one after NO release.


 Please wait while we load your content...
Please wait while we load your content...