Retracted Article: Synthesis of triazolidine-3-one derivatives through the nanocellulose/hydroxyapatite-catalyzed reaction of aldehydes and semicarbazide†
Abstract
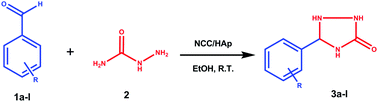
A novel nanocellulose/hydroxyapatite heterogeneous catalyst was used in the one-pot, two-component synthesis of triazolidine-3-one derivatives, produced from the cyclocondensation reaction between semicarbazide and an aromatic aldehyde. With the use of ethanol solvent, the products formed after a short reaction time (<30 min). The catalyst was characterized by XRD, TEM, SEM-EDS and BET analysis. The main advantages of this protocol were excellent yields (90–96%) of the products, mild and environmentally friendly reaction conditions, good atom-economy, and a simple and fast work-up with the added benefit of a recyclable catalyst, negating the need of chromatographic separations of the intermediates. The twelve triazolidine-3-one derivatives (3a–l) were characterized by 1H, 13C and 15N NMR, FT-IR, and HR-MS and represented a new family of compounds containing the 1,2,4-triazolidin-3-one moiety.


 Please wait while we load your content...
Please wait while we load your content...