Color-tunable entangled coordination polymers based on long flexible bis(imidazole) ligands and phenylenediacetate†
Abstract
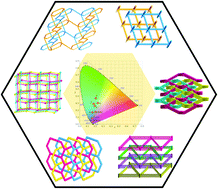
Six coordination polymers, namely, [Co2(mpda)2(Hmpda)(bibp)]·H2O (1), [Pb(Hmpda)(bibp)Cl]·H2O (2), [Cd(mpda)(bibp)] (3), [Cd(ppda)(bibp)] (4), [Cd(opda)(bib)0.5(bipy)]·2H2O (5), and [Zn(opda)(bib)] (6), where H2pda = phenylenediacetic acid, bibp = 4,4′-bis(imidazol-1-ylmethyl)biphenyl, bib = 1,4-bis(imidazol-1-ylmethyl)benzene, and bipy = 2,2′-bipyridine, have been synthesized using different metal salts with phenylenediacetic acid and bis(imidazole) ligands under hydrothermal conditions. Their structures have been characterized by elemental analysis, IR spectroscopy, single-crystal X-ray crystallography and powder X-ray diffraction (PXRD) analysis. Owing to the coordination geometry around the metal ions and the diverse coordination modes of the flexible ligands, the obtained complexes 1–6 display different types of entanglement. Complex 1 possesses an extended 2D polyrotaxane and polycatenane network based on 1D ringed ribbon-like chains. The 2D coordination layers of 2 and 4 are entangled to produce 2D → 3D polythreaded architecture. Both 3 and 6 are composed of three-fold interpenetrating 3D diamond frameworks containing single chiral helical chains, whereas three 2D helical layers of 5 interpenetrate each other in an alternating arrangement of three-stranded right- and left-handed helices. Furthermore, the fluorescence emission color of complexes 2–6 could be tuned from dark blue to light blue and green.


 Please wait while we load your content...
Please wait while we load your content...