Probing the formation of supramolecular assemblies of amphiphilic N-methyl glycine and N,N dimethyl β-alanine derivatives†
Abstract
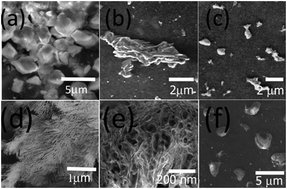
Supramolecular assemblies were prepared using new amphiphilic dervivatives of N-methyl gylcine and N,N dimethyl-β-alanine. Both N-methylated amino acids were conjugated with 1,4 diamino butane to form an amphiphile and a bolaamphiphile each. For N-methyl glycine we designed a monomer N-(4-aminobutyl)-2-(methylamino acetamide) (AMA) and a dimer N,N′-(butane-1,4-diyl)bis(2-(methylamino) acetamide) (BMA). Similarily for N,N dimethyl β-alanine, we prepared a monomer N-(4-aminobutyl)-3-(dimethlyamino) propanamide (ADP) and a dimer N,N′-(butane-1,4-diyl)bis(3-(dimethylamino)propanamide) (BDP). The formation of the assemblies was investigated at a pH range of four through eight over a period of three weeks. The growth of the assemblies was examined by dynamic light scattering analysis and the morpholgies obtained were probed by scanning electron microscopy. Our results indicated that the formation of assemblies was dependent on the structure (monomer or dimer) as well as pH. In general, we observed the formation of nano and microfibers, microtubules, micelles or unique vesicular structures. Specifically, it was observed that the dimers had a higher tendency toward the formation of fibrillar structures under neutral conditions. The structural transformations of the assemblies was probed by FTIR and CD spectroscopy. DSC analysis revealed that the assemblies in general began unfolding in the temperature range of 70–80 °C. Furthermore, the assemblies were found to have no cytotoxicity to mammalian fibroblasts. Such assemblies may be potentially applicable in biomedical applications such as drug delivery or tissue regeneration.


 Please wait while we load your content...
Please wait while we load your content...