Synthesis of ferrocenylmethylidene and arylidene substituted camphane based compounds as potential anticancer agents†
Abstract
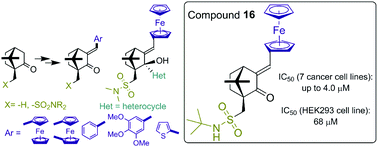
Herein is described the synthesis of (+)-camphor derivatives containing sulfonamide groups, ferrocenylmethylidene or arylidene moieties. The obtained derivatives were tested against seven human cancer cells lines, namely BV-173, K-256a, NB-4, A549, H1299, MCF-7, and MDA-MB231, and two normal human cell lines, HEK293 and HUVEC, in order to determine their activity against malignant cells. Some of them exhibit IC50 values below 10 μM in at least one of the cancer cell lines. Ferrocenylmethylidene ketone 16 can be outlined as the most potent and selective in the current study (IC50 for cancer cells – up to 4.0 μM; IC50 for HEK293 and HUVEC – 68 and 69 μM, respectively). There is a clear trend showing that the presence of a conjugated ferrocenylmethylidene group is essential for the cytotoxicity, however different sulfonamide substituents and derivatization of the carbonyl group can modify the activity. Thus, this class of compounds could have good prospects for further structural optimisation.


 Please wait while we load your content...
Please wait while we load your content...