An alternative method to access diverse N,N′-diquaternised-3,3′-biquinoxalinium “biquinoxen” dications†
Abstract
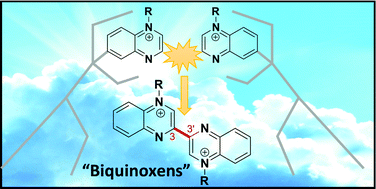
An alternative synthetic route for the design of N,N′-diquaternised-3,3′-biquinoxalinium “biquinoxen” dications is reported, involving oxidative radical coupling of dithionite reduced quinoxaline quaternary salts. Although the reaction is not regioselective, leading to relatively modest yields (up to 32%), the advantages of this new synthetic protocol lie in a simple potentially gram scale synthesis using inexpensive easily accessible reagents with no metal catalysts and no purification steps. Thus whereas the method reported previously to access the N,N′-dimethyl-3,3′-biquinoxalinium, “methylbiquinoxen” precursor gave higher yield than the new method reported here, this new method avoids the limitation of using scarce oxonium reagents. Overall, the new protocol is a robust synthetic strategy which offers new design possibilities.


 Please wait while we load your content...
Please wait while we load your content...