A series of novel cadmium(ii) coordination polymers with photoluminescence and ferroelectric properties based on zwitterionic ligands†
Abstract
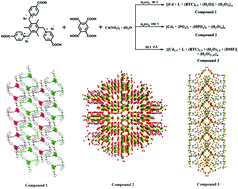
A series of Cd(II) compounds, abbreviated as {[Cd·L·(BTC)0.5·H2O]·(H2O)3}n (1), [Cd5·(PO4)2·(HPO4)2·(H2O)4]n (2) and {[Cd2.5·L·(BTC)1.5·(H2O)1.5·(DMF)]·(H2O)2.35}n (3), were obtained by using a zwitterionic ligand (1,1′,1′′-(2,4,6-trimethybenzene-1,3,5-triyl)-tris(methylene)tris(4-carboxypyridinium) tribromide, H3LBr3) and an aromatic secondary ligand (H4BTC). Structure determination confirmed that compounds 1 and 3 possess the structure of 1D and 2D metal–organic frameworks respectively while compound 2 belongs to a novel inorganic crystal, which may be produced by introducing different categories and concentrations of inorganic acids. In addition to reasonable thermal stability (up to 300 °C), the titled Cd(II) compounds exhibited different photoluminescence emissions in the solid state resulting from coordination environments and crystal structures. Moreover, compound 3 also exhibited a ferroelectric behavior with a saturated spontaneous polarization (Ps) of 0.20 μC cm−2, indicating that compound 3 is a potential ferroelectric material at room temperature.


 Please wait while we load your content...
Please wait while we load your content...