POCl3-mediated cyclization of (+)-S-mahanimbine led to the divergent synthesis of natural product derivatives with antiplasmodial activity†
Abstract
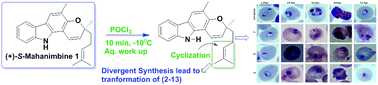
(+)-Mahanimbine (1) is a carbazole alkaloid isolated from Murraya koenigii. It undergoes rearrangements under thermal, photolytic, or acidic conditions and is transformed into diverse structural motifs; most of its derivatives have also been reported as natural products with distinct biological activities. Herein, we report the POCl3-mediated reaction of (+)-S-mahanimbine, which rearranged and transformed into seven new and five known natural products viz. isocyclomahanimbine (3), curryanin (5), bicyclomahanimbine (9), curryangin (10) and murrayazolinine (13), which includes previously undisclosed chemistry. The compounds’ (1–13) structural assignments were authenticated by extensive 1D and 2D NMR experiments, HRESIMS, and comparison with the literature data. Compound 3 was unambiguously confirmed by X-ray crystal diffraction analysis. Compounds 1–13 were screened for the first time for anti-parasitic activity against Plasmodium falciparum, in which the new compounds 2, 6 and 7 were proven to be the most potent and exhibited the highest antiplasmodial activity with IC50 values of 2.7, 4.5 and 3.2 μM, respectively. These findings may provide new insights into the design of new pyrano carbazole alkaloid derivatives with promising antiplasmodial potential.


 Please wait while we load your content...
Please wait while we load your content...