Magnetic nanoparticle-supported DABCO tribromide: a versatile nanocatalyst for the synthesis of quinazolinones and benzimidazoles and protection/deprotection of hydroxyl groups†
Abstract
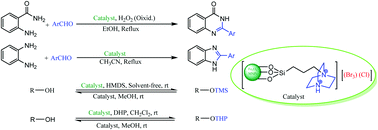
1,4-Diazabicyclo[2.2.2]octane tribromide supported on magnetic Fe3O4 nanoparticles (MNPs-DABCO tribromide) as a novel heterogeneous tribromide type compound was found to be an efficient and reusable nanocatalyst for the one-pot synthesis of 2-arylquinazolin-4(3H)-ones and 2-aryl-1H-benzo[d]imidazoles through oxidative cyclization of aldehydes with 2-aminobenzamides and 1,2-phenylenediamine, respectively. Also, MNPs-DABCO tribromide catalyzed trimethylsilylation/tetrahydropyranylation and desilylation/depyranylation of a wide variety of alcohols and phenols through changing the solvent medium at room temperature.


 Please wait while we load your content...
Please wait while we load your content...