Organic superbase derived ionic liquids based on the TFSI anion: synthesis, characterization, and electrochemical properties†
Abstract
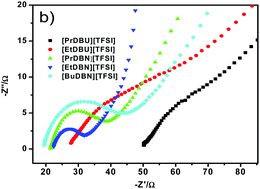
A series of hydrophobic room temperature ionic liquids (RTILs) composed of organic superbase-derived cations and the TFSI anion (bis(trifluromethylsulfonyl)imide) were designed and synthesized. The structures of these new RTILs were determined using 1H-NMR, 13C-NMR, IR, and elemental analysis. Their physicochemical properties, including thermal properties, density, viscosity, and electrochemical properties, were investigated. Thermogravimetric analysis (TGA) revealed that these superbase-derived RTILs have high decomposition temperatures of up to 450 °C. Studies on their electrochemical properties demonstrated that these RTILs have relatively wide electrochemical windows of up to 4.6 V and good ionic conductivities at room temperature. Their application potential as novel electrolytes in supercapacitors was also evaluated.


 Please wait while we load your content...
Please wait while we load your content...