MCM-41-supported phosphotungstic acid-catalyzed cleavage of C–O bond in allyl aryl ethers†
Abstract
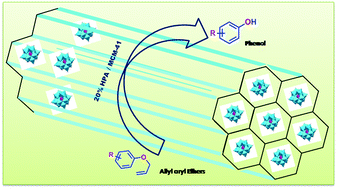
Removal of the protecting allyl group from allyl aryl ethers in the presence of other oxygen protecting groups was successfully achieved using a solid acid supported on the high surface area material MCM-41. The catalyst showed excellent activity in the presence of various electron withdrawing, electron donating, and oxidizable functional groups. The methodology is also very useful for the removal of protecting allyl groups of various natural products such as vanillin, isovanillin, and other oxygen functionalized aldehydes and ketones.


 Please wait while we load your content...
Please wait while we load your content...