Resolving synthetic challenges faced in the syntheses of asymmetric N,N′-ethylene-bridged energetic compounds†
Abstract
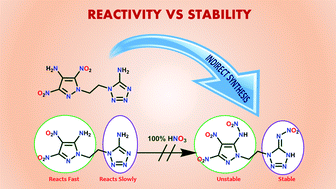
Synthetic challenges faced during the syntheses of asymmetric N,N′-ethylene-bridged energetic compounds due to the differences in the reactivity and stability of various types of energetic rings are addressed. The elusive asymmetric compounds, 12 (in which nitramino-pyrazole is bonded to nitroimino-tetrazole via an N,N′-ethylene-bridge), and 15 and 17 (in which azido-pyrazole is bonded to nitroimino-tetrazole via an N,N′-ethylene-bridge), were obtained in good yields. All the compounds were thoroughly characterized using IR, NMR [1H, 13C{1H}, 15N], elemental analysis and differential scanning calorimetry (DSC). The structures of 13 and 14 were further confirmed via X-ray crystal analysis. Heats of formation and the detonation properties of all the energetic compounds were calculated using Gaussian 03 and EXPLO5 v6.01 programs, respectively. Impact and friction sensitivities were determined using the standard BAM technology.


 Please wait while we load your content...
Please wait while we load your content...