Hydrazine-assisted electrolytic hydrogen production: CoS2 nanoarray as a superior bifunctional electrocatalyst†
Abstract
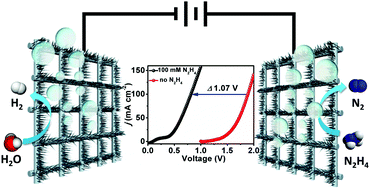
The sluggish kinetics of the oxygen evolution reaction has severely hindered the energetic convenience of electrolytic water splitting. Herein, we demonstrate that a CoS2 nanoarray on Ti mesh (CoS2/TiM) behaves as an efficient and durable catalyst for the hydrazine oxidation reaction in 1.0 M KOH with 100 mM hydrazine, which requires the potential of 125 mV to achieve 100 mA cm−2. The high hydrogen-evolving activity of CoS2/TiM makes it a bifunctional catalyst for energy-saving electrolytic hydrogen generation by replacing water oxidation with hydrazine oxidation. To drive 100 mA cm−2, its two-electrode electrolyzer demands a low cell voltage of only 0.81 V and it exhibits remarkable long-term electrochemical durability and nearly 100% Faradic efficiency for hydrogen evolution.


 Please wait while we load your content...
Please wait while we load your content...