Design and in vitro biological evaluation of substituted chalcones synthesized from nitrogen mustards as potent microtubule targeted anticancer agents†
Abstract
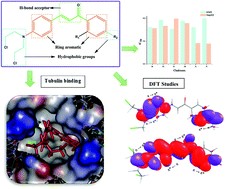
A new series of p-[N,N-bis(2-chloroethyl)amino]benzaldehyde substituted chalcone derivatives were designed and synthesized, and their structures were characterized by spectroscopic techniques and single crystal XRD studies. Compounds 3a–f crystallized in the triclinic system with a centrosymmetric space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , except for crystal 3c which crystallized in the monoclinic crystal system with a centrosymmetric space group P21/c. Molecular docking studies were utilized to reveal the binding mode of the derivatives to identify new tubulin inhibitors. Density functional theory calculations were performed to understand the structural and electronic properties of these chalcones. The DFT results show that the HOMOs of all the chalcones lie in the range of −5.65 to −6.17 eV and the LUMOs in the range of −2.01 to −3.21 eV. The experimental results are well supported by the theoretical structural analysis. The biological activity of these compounds showed high potency of growth inhibitory effects with sub-micromolar IC50 values ranging from 0.089 to 0.200 μM against A549 and HepG2 cancer cell lines. Furthermore, these compounds exhibited a strong inhibitory effect on tubulin polymerization. 3e showed the highest mean activity against both the cancer cells and in tubulin inhibition. This correlated well with the theoretical results from the pharmacophore binding model. Hence, these six compounds, particularly 3e, could be considered as potential leads in the development of new anticancer agents.
, except for crystal 3c which crystallized in the monoclinic crystal system with a centrosymmetric space group P21/c. Molecular docking studies were utilized to reveal the binding mode of the derivatives to identify new tubulin inhibitors. Density functional theory calculations were performed to understand the structural and electronic properties of these chalcones. The DFT results show that the HOMOs of all the chalcones lie in the range of −5.65 to −6.17 eV and the LUMOs in the range of −2.01 to −3.21 eV. The experimental results are well supported by the theoretical structural analysis. The biological activity of these compounds showed high potency of growth inhibitory effects with sub-micromolar IC50 values ranging from 0.089 to 0.200 μM against A549 and HepG2 cancer cell lines. Furthermore, these compounds exhibited a strong inhibitory effect on tubulin polymerization. 3e showed the highest mean activity against both the cancer cells and in tubulin inhibition. This correlated well with the theoretical results from the pharmacophore binding model. Hence, these six compounds, particularly 3e, could be considered as potential leads in the development of new anticancer agents.


 Please wait while we load your content...
Please wait while we load your content...