Low temperature decomposition of ozone by facilely synthesized cuprous oxide catalyst†
Abstract
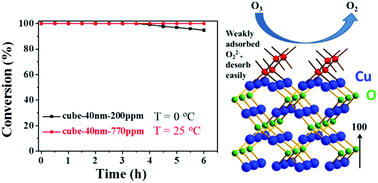
In recent years, ozone remains to be one of the most common air pollutants generated by ultraviolet irradiation and/or emitted from daily machines. Since ozone is harmful to both humans and the environment even in trace concentrations, it is challenging to catalytically decompose ozone with high efficiency and low cost. In this study, surface exposure and particle size-controlled cuprous oxide (Cu2O) is synthesized in ambient conditions through a simple reductive solution chemistry route. Ozone decomposition test shows that 40 nm cubic Cu2O has 100% degradation efficiency at 25 °C, with high resistance to water vapor. Moreover, the high catalytic activity can also be maintained for >6 h at 0 °C. The related mechanism is found to be a facile desorption of the weakly adsorbed O22− intermediate on {100} plane of cubic Cu2O in the ozone decomposition process. This is proven by oxygen temperature programmed desorption, X-ray photoelectron spectroscopy and atomic model establishments. The results provide promising evidence of the facilely synthesized Cu2O catalyst in a highly active ozone degradation process, for the benefit of human health and environment sustainability.


 Please wait while we load your content...
Please wait while we load your content...